Hedgehog diffractometer "Igel" D6 for neutron diffraction using a modified Laue method
- maker:
- Institut Laue-Langevin

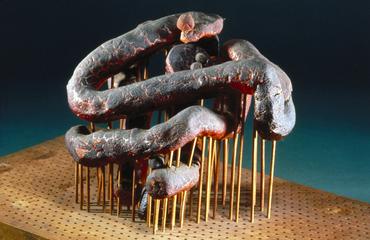
The “Igel” D6 diffractometer (nicknamed the ‘hedgehog’) was built in 1970 by the Institut Laue-Langevin in Grenoble, France.
This spherical instrument is approximately 1.7 metres in diameter and weighs between one to two tons. The external part of the diffractor is covered in a series of approximately 100 cylinders which mount the detectors which line the interior of the device.
This “Igel” D6 diffractometer (nicknamed the ‘hedgehog’) was built in 1970 by the Institut Laue-Langevin, a joint British- French-German research institute based in Grenoble, France. This centre specialises in the study of neutrons and used the diffractometer themselves to analyse the structures of crystalline solids through a technique called diffraction.
Diffraction was developed by German scientist Max von Laue in the early 20th century, for which he received a Nobel prize in in 1914. Von Laue’s original method of diffraction involved colliding x-rays into the substance and observing and recording how these x-rays would scatter. Notably, von Laue’s method allowed the results to be captured on special x-ray scans, making them easier to visually record the different structures of crystalline solids.
This D6 multiple diffraction spectrometer used a modified version of Laue’s method which uses neutrons instead of x-rays to analyse the structures of many single crystals simultaneously. Neutrons produced in a nuclear reactor can be slowed by graphite control rods to the point that they travel around the same speeds and at the same wavelengths of x-rays. This allows neutrons to be used in diffraction analyses, with results being captured on film.
Neutron and x-ray diffraction are still used today, and whilst each method has its own strengths and limitations, they are generally considered to be complementary methods which are suited to analysing different molecules. A key difference between the two is that neutrons are scattered through interactions with the nuclei of atoms, and x-rays are scattered by the electrons of atoms. This means that neutron diffraction is more heavily shaped by the properties of the nuclei of the crystals, whilst x-ray diffraction is shaped more by the atomic number of the crystals. Another practical difference between the two is that neutrons are capable of higher penetration depth which allows neutron diffractors (such as the “Igel” D6) to analyse multiple crystals at the same time in bulk but requires a supply of neutrons which must come from a nearby nuclear reactor.
Details
- Category:
- Experimental Chemistry
- Object Number:
- 1982-788
- Measurements:
-
overall: 2.2 m 2 mm, 2.5 t
- type:
- diffractometers
- credit:
- Institut Laue-Langevin




