Vial of DPT Vaccine, Lyons, France, 1994
- maker:
- Pasteur Mérieux


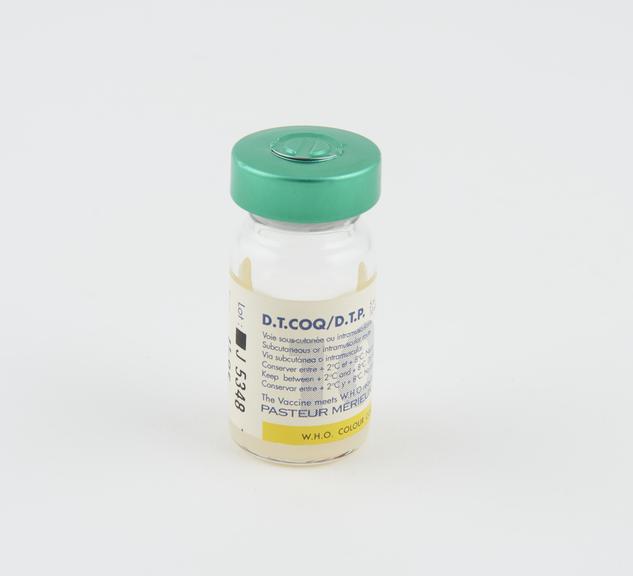
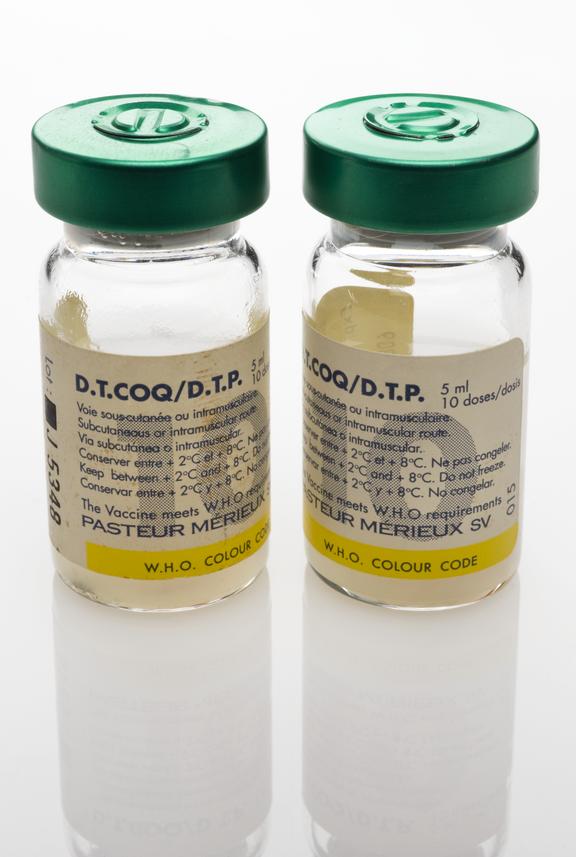
One of two glass vials, each containing 5ml (10 doses) of D.P.T. vaccine for subcutaneous or intramuscular injection to provide protection against Diphtheria, Tetanus and Pertussis ( Whooping Cough), as part of the UNICEF/WHO Extended Programme on Immunisation (EPI), manufactured by Pasteur Merieux, Lyon, France, 1994
This glass vial contains 5ml of the DPT vaccine – the equivalent of ten doses. The vaccine was injected into a human patient to gain immunity against the potentially fatal diseases Diphtheria, Tetanus and Pertussis (Whooping Cough). It was administered as part of the United Nations Children’s Fund (UNICEF) and World Health Organisation (WHO) Extended Programme on Immunisation (EPI). Diphtheria is now rare in the UK. This is because of vaccination programmes which began in 1940, when the death rate from diphtheria was high. Diphtheria is still a common disease in parts of the world. The vaccine was manufactured by Pasteur Merieux, in France.
The EPI targeted six main childhood diseases and immunisation in the developing world was seen a particularly referenced. This is important because vaccine-preventable diseases disproportionately affect the poorest section of the developing world. This form of DTP vaccine caused controversy in the 1990s as it resulted in adverse reactions in a small proportion of children. These safety concerns led to the combined vaccination being withdrawn in 1997, to be replaced with DTaP, a modified version of the vaccine.
Details
- Category:
- Public Health & Hygiene
- Object Number:
- 1994-169/2
- Materials:
- glass, paper (fibre product), aluminium (metal) and materia medica
- Measurements:
-
overall: 44 mm 20 mm, .015kg
- type:
- vaccine