



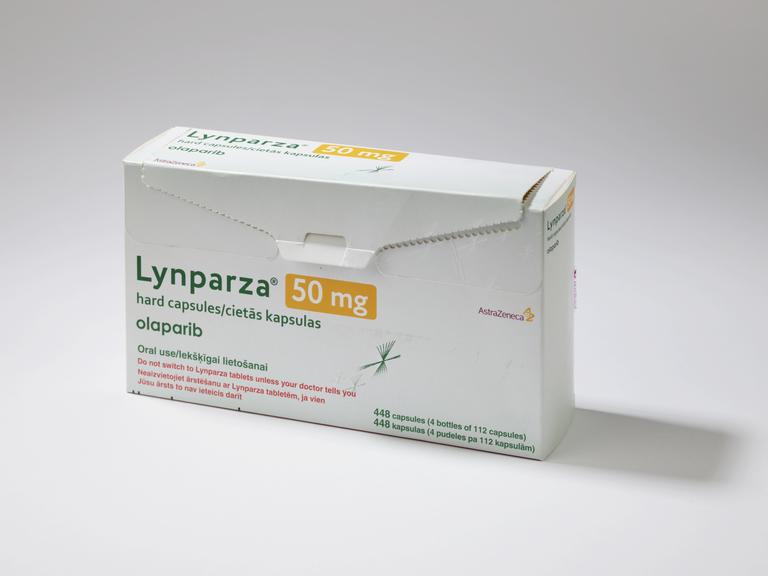

Empty packaging for Lynparza, the brand name of the drug Olaparib, the first PARP inhibitor drug using a technique known as ‘synthetic lethality’ used for targeting treatment for people with BRCA gene mutation type cancers, made by AstraZeneca, United Kingdom, 2019
Scientists and clinicians at The Institute of Cancer Research and The Royal Marsden worked together for over ten years to develop this world first medicine.
PARPS ((Poly(ADP-ribose) polymerases) are proteins that cells, including some cancer cells, need to repair themselves in order to multiply. By blocking these proteins, cancer cells die, leaving healthy ones alone. Researchers found that this treatment was effective for people who had cancers caused by changes to their BRAC genes. BRAC1 and BRAC2 genes protect us from breast and ovarian cancers. Olaparib was the start of the idea of ‘treating cancer by targeting a weakness’ in a process known as synthetic lethality.
After clinical trials proved successful, Olaparib was approved in Europe and the United States in 2014. It was the first ever cancer treatment targeted against an inherited genetic fault to be licensed. Unlike common types of chemotherapy, which is infused, this targeted cancer treatment is taken as tablets or capsules once or twice a day.
Details
- Category:
- Materia Medica & Pharmacology
- Object Number:
- 2020-147
- Materials:
- cardboard
- Measurements:
-
overall: 100 mm x 340 mm x 50 mm,
- type:
- packaging
- credit:
- Professor Christopher Lord, Dr Rachel Bough, Dr Stephen Pettitt, The Institute of Cancer Research, London




