Reveal™ XT kit for an insertable cardiac monitor
Boxed Reveal™ XT kit including Reveal™ XT insertable cardiac monitor (ICM) also known as an implantable loop recorder, model number 9529, with sterile packaging, made in the United States, April 2011
More
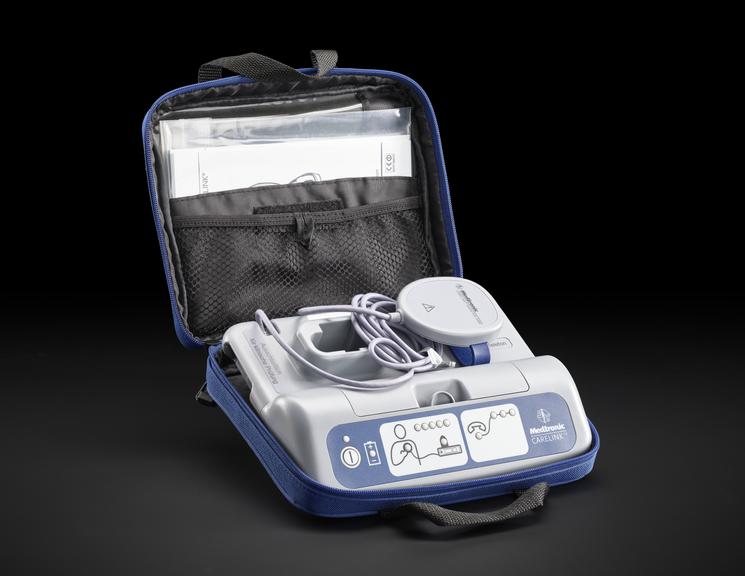
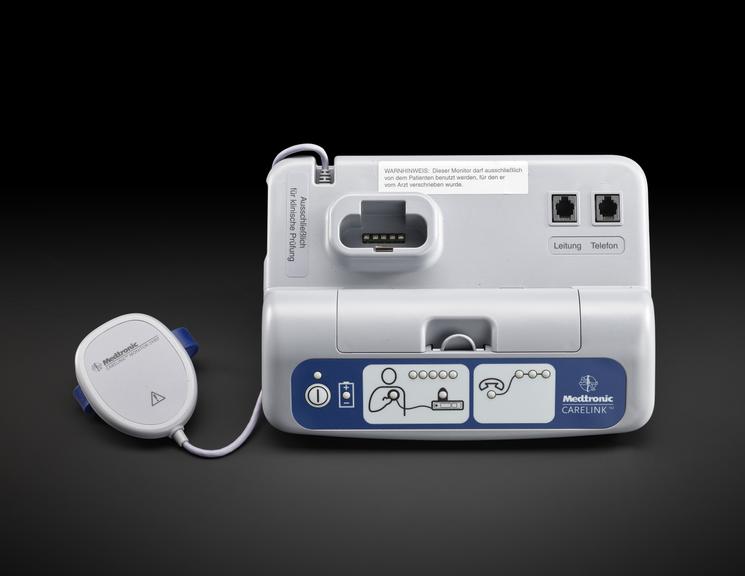
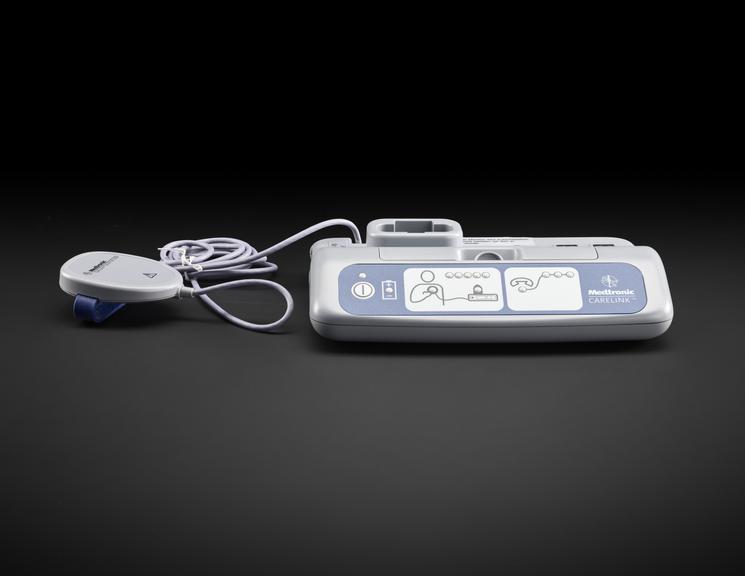
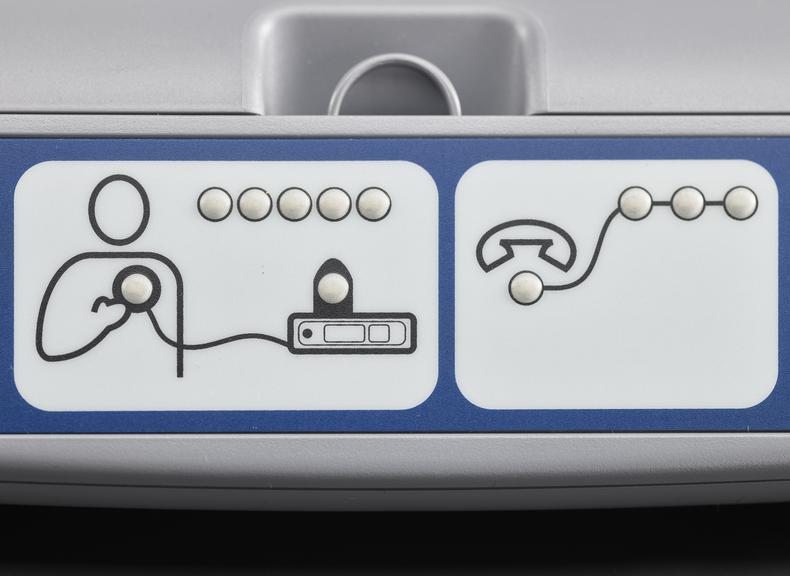
For some people a single ECG or 24 hour monitor may not capture an irregular heart rhythm that causes them to faint. Episodes can be unpredictable and sometimes without symptoms. Fear of an episode can disrupt every-day life and can be emotionally testing. Insertable cardiac monitors, also known as implantable loop devices, continuously loop its memory and has automatic triggers to store irregular heart beat events. Episodes can also be logged by the user and all data is uploaded for remote monitoring. The wireless device is implanted under the skin during a small operation involving a local anaesthetic. To record an event, a person placed a hand held device over their cardiac monitor, and presses a button. Later their doctor analyses the stored information and determines if the episode was caused by an irregular heart rhythm.
Medtronic has been a market leader in this area since the launch of their first device in 1998. The Reveal™ XT was launched in 2009 with improvements in accuracy and software updates. With a battery life of three years, the device could record person activated and automatically detected events. The software included the world's first atrial fibrillation detection algorithm
- Materials:
- titanium , polyurethane , silicon , parylene and plastic
- Object Number:
- 2022-627/1
- Image ©
- The Board of Trustees of the Science Museum