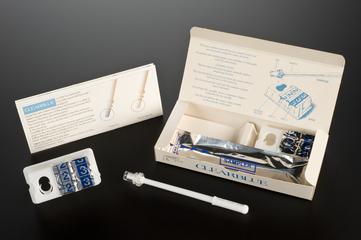
Rapid-i stick and RapidStraw, vitrification device for storing human eggs, used within mitochondrial donation therapy, c.2023
- Made:
- circa 2023 in unknown place
Rapid-i stick and RapidStraw vitrification device for storing human eggs and embryos, used by the fertility team at the Newcastle Fertility Centre to carry out mitochondrial donation therapy (MDT), made by Vitrolife, c.2023. Consists of plastic 'straw' and plastic storage pipette that has a small opening enough to hold an egg or embryo. The pipette is then sealed within the straw before being placed in liquid nitrogen for storage.
The Rapid-i stick is a plastic rod with a small hollow channel at one end just big enough to hold and store a human egg or embryo within fertility treatment. The size of the pipette's opening diameter and the design of its smooth edges means eggs and embryos can be handled carefully without harming them. The pipette is then sealed within RapidStraw, a plastic 'straw' containing supercooled air before being placed in liquid nitrogen for storage. It is kit that enables embryos and eggs to be simply handled, stored and transported without the risk of contamination. The device is made by Vitrolife.
It was donated and used by a team at the Newcastle Fertility Centre who have developed techniques for a fertility treatment called mitochondrial donation treatment (MDT). This aims to prevent children inheriting rare but serious diseases caused by faulty mitochondria. This therapeutic treatment has led to the phrase 'three-parent babies'. In the UK, about one in 6,000 babies are affected by mitochondrial disorders.
Found in nearly every cell in the body, thousands of mitochondria are dotted around a cell’s nucleus. They produce energy for the cell to function. When a person has a mitochondrial disease, the mitochondria in the cells don’t produce enough energy. The parts of the body commonly affected are those that have the highest energy demands, such as brain, muscle, liver, heart and kidney.
Mitochondrial donation treatment (MDT) involves combining both parents nuclear genetic material and placing it within a donor egg with healthy mitochondria, to avoid the risk of passing on faulty mitochondria. The Newcastle clinic became the first and only national centre licensed to perform MDT, with the first cases approved in 2018. It was reported in 2023 that the first babies had been born using this technique.
Details
- Category:
- Obstetrics, Gynaecology & Contraception
- Object Number:
- 2024-428
- Materials:
- plastic (unidentified)
- credit:
- THE NEWCASTLE UPON TYNE HOSPITALS NHS FOUNDATION TRUST