Professor Sarah Gilbert's doorplate
Professor Sarah Gilbert’s doorplate from her office at the Jenner Institute, University of Oxford
More
Sarah Gilbert has been studying viruses and vaccines as ways to prevent them for her entire scientific career. Building on her years of experience, Professor Gilbert was able to quickly put that knowledge to use becoming the co-developer and Project Lead for Oxford’s COVID-19 vaccine.
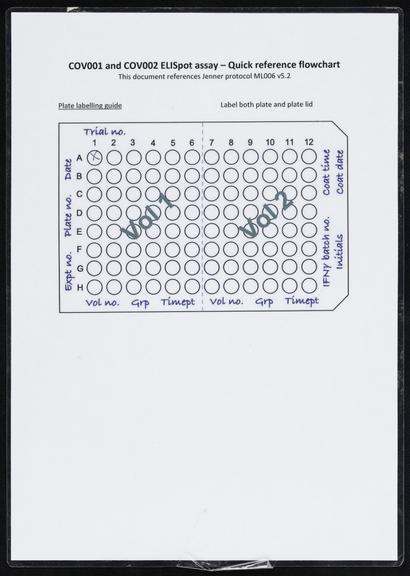
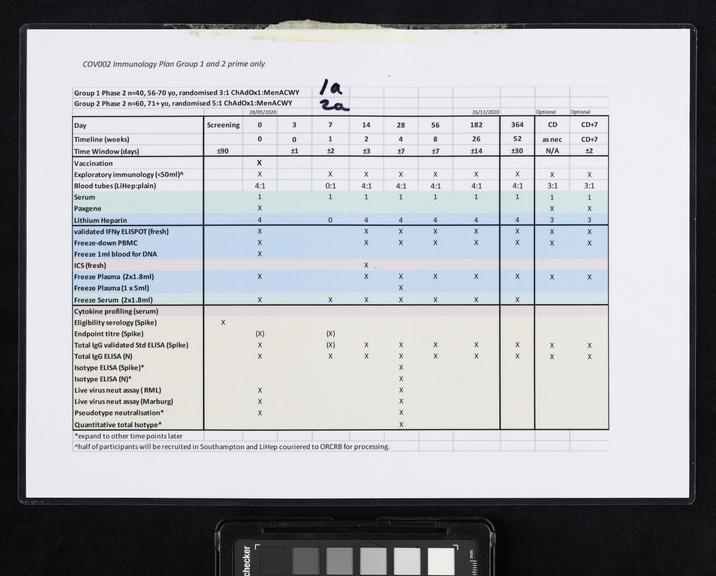
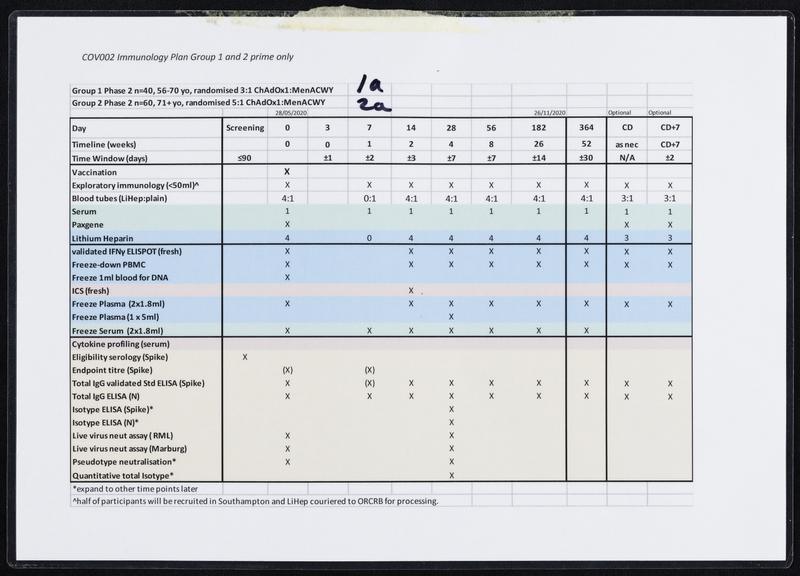
Once the genetic code of COVID-19 was released on 12 January 2020, scientists including Sarah Gilbert, Professor Vaccinology, at the University of Oxford, immediately set to work, on designing a vaccine. On the 6th of March 2020, the Production Team started small-scale manufacture of the Oxford team’s vaccine known as ChAdOx1 nCoV-19. After several stages of cell growth, harvesting and purification the first vials were filled on 2nd April 2020. After rigorous checks, the first volunteers were injected with the vaccine on 23 April 2020. On 30 April 2020, Oxford University signed a deal with pharmaceutical company AstraZeneca to mass-produce the vaccine. This included a clause that no profit would be made from providing the vaccine to low-income countries. Large scale clinical trials followed before the vaccine was approved by the Medicines and Healthcare products Regulatory Agency (MHRA) on 30th December 2020. 5 days later, Brian Pinker was the first person to receive a dose of the Oxford/AstraZeneca vaccine. Over 3 billion doses have been delivered worldwide.
In 2021, Professor Gilbert was awarded an DBE for services to Science and Public Health, and was honoured alongside many of her colleagues. Her book ‘Vaxxers: The Inside Story of the Oxford AstraZeneca Vaccine and the Race Against the Virus’, details her work and those of the team around her.