
Thalomid (thalidomide) capsules
- Made:
- 1999 in United States






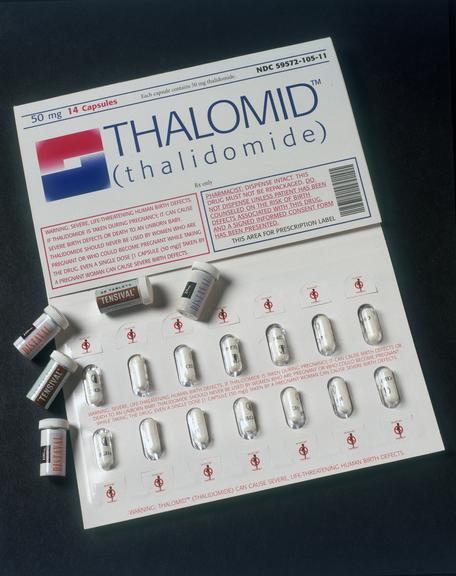


THALOMID (TM) (thalidomide) in blister pack marketed for use in treating leprosy, HIV infection and some cancers under strictly controlled conditions, 1999
Thalidomide is prescribed by the National Health Service for the symptoms of myeloma, a blood cancer. Its use is tightly controlled. People taking thalidomide must use birth control and undergo regular pregnancy testing. It is not recommended for people who are breastfeeding, who are pregnant or who are planning to start a family. It was also trialled as a treatment for HIV and Hansen's disease (leprosy) in the mid 1980s.
If taken during the first three months of pregnancy, thalidomide causes impairments such as limb difference, sight loss, hearing loss, facial paralysis, and impact to internal organs. A single tablet can be the cause. It took three years for the link to be made between thalidomide and the impairments it caused. In March 2021, 452 people are living with thalidomide impairments in the United Kingdom. No two of them are the same. It is estimated that up to 12,000 families in the United Kingdom may have been impacted and up to 147,000 worldwide.
UK distributors withdrew the drug in 1961 and a government warning was issued in May 1962. The compound thalidomide was created in 1954 by Chemie Grünenthal, a German pharmaceutical company. It was first officially released in West Germany for use as a sedative with minimal side-effects in October 1957. Thalidomide was distributed in the United Kingdom by Distillers, a drinks company.
Thalidomide was used in various drug preparations under several different names. Although today it is associated primarily as a treatment for pregnancy related nausea, it was also prescribed to anyone experiencing symptoms of colds, flu, headaches, anxiety, and insomnia.
Thalidomide’s impact rippled from people’s births shaping their lives and those around them. A study by the Thalidomide Trust found that 80% of people are living with chronic pain because of wear and tear on their bodies. Many have become ambassadors for disability awareness, not because they wanted to, but because they had to, often facing prejudice and exclusion from society.
Details
- Category:
- Materia Medica & Pharmacology
- Object Number:
- 2000-590
- Materials:
- card
- Measurements:
-
overall: 132 x 213 x 13 mm
- type:
- thalidomide
- credit:
- Celgene Corporation




