
Packaging for Paxlovid
- Made:
- 2022 in Germany and United Kingdom
- maker:
- Pfizer Limited and National Health Service













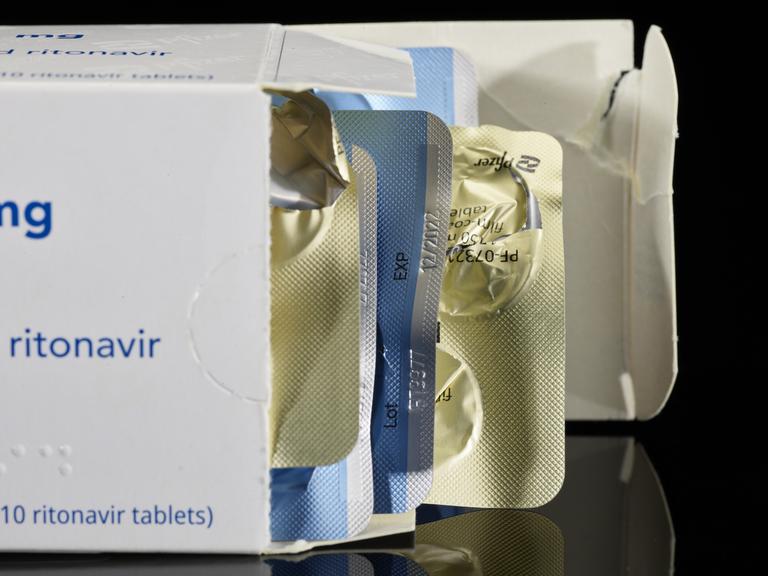













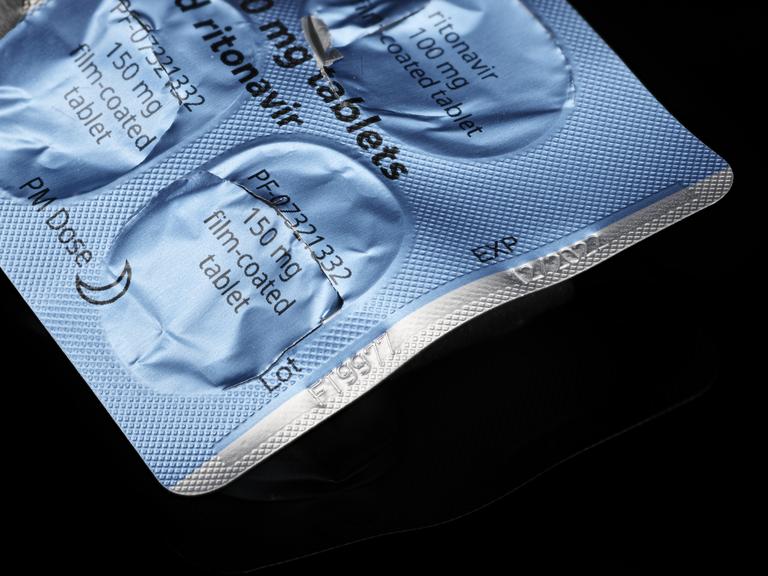
Postal envelope, plastic bag, NHS and UK Health Security Agency leaflet, package leaflet, packaging, and 5 empty blister packets (expiry date 12/2022, lot FT9977) for Paxlovid, an antiviral medicine used to treat COVID-19 in high risk patients, May 2022
When the COVID-19 pandemic first began in 2019, there was a limited understanding of how best to treat it. However, scientists have since developed a variety of different treatment methods.
Pfizer began developing an antiviral medication to treat COVID-19 in March 2020. However, they were not starting from scratch. In 2003, Pfizer had developed an antiviral that targeted the coronavirus that caused severe acute respiratory syndrome (SARS), but the outbreak was contained before it could be tested in patients. By adapting this medication, they were able to develop Paxlovid which began clinical trials in August 2021. Paxlovid works by stopping the virus that causes COVID-19 multiplying in our cells and our bodies. It was developed to try to prevent high risk patients needing to go to hospital. During the clinical trials, Paxlovid was found to reduce the risk of hospitalisation and death by 88%. In the UK, it was approved for use in December 2021 for people most at risk from COVID-19.
Details
- Category:
- Materia Medica & Pharmacology
- Object Number:
- 2023-105
- Materials:
- paper (fibre product), plastic (unidentified), cardboard and foil
- Measurements:
-
overall (packaging): 62 mm x 111 mm x 55 mm,
overall (postal envelope): 295 mm x 195 mm x 10 mm,
overall (blister packet): 60 mm x 105 mm x 10 mm,
overall (package leaflet): 295 mm x 180 mm
overall (information leaflet): 210 mm x 148 mm
overall (plastic bag): 242 mm x 150 mm
- type:
- drug packaging

