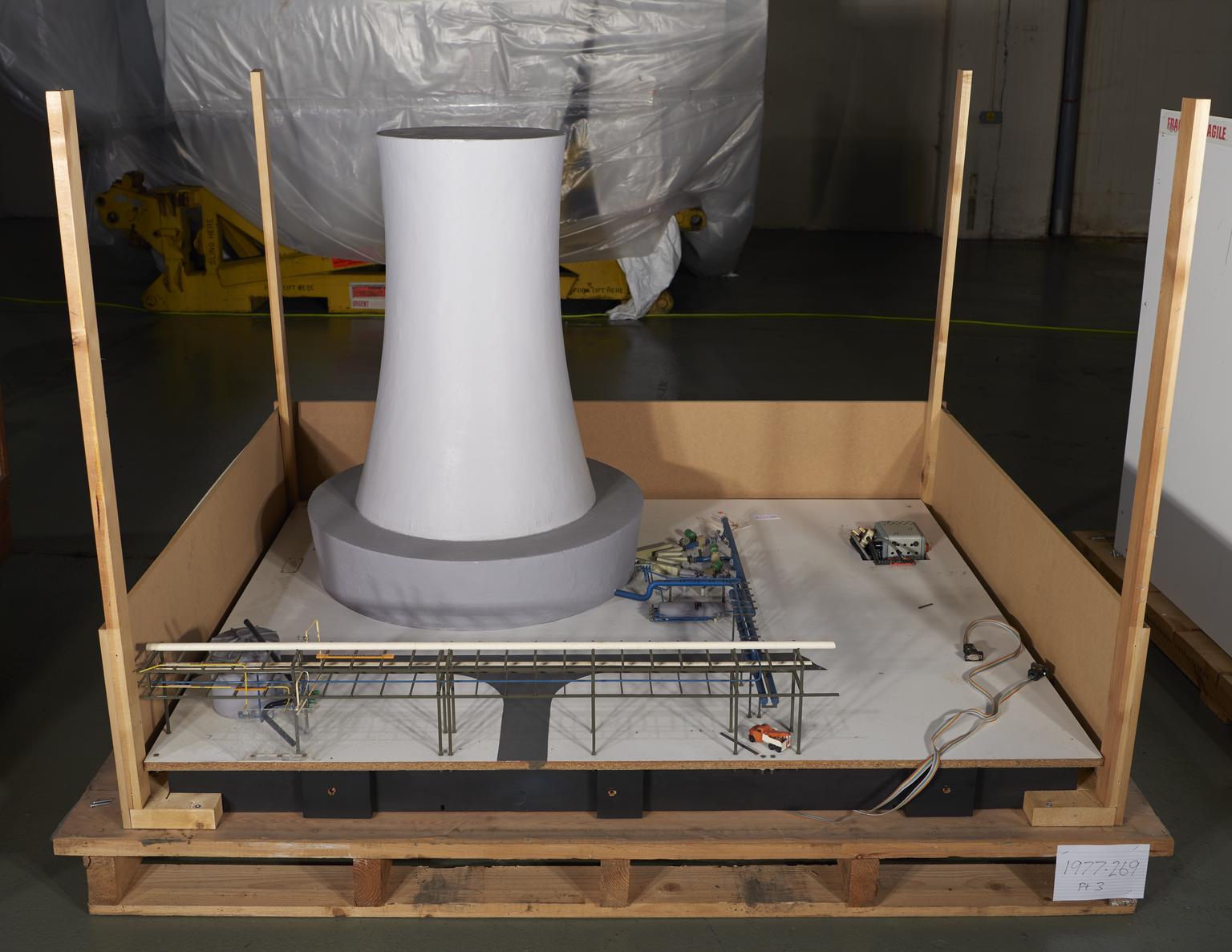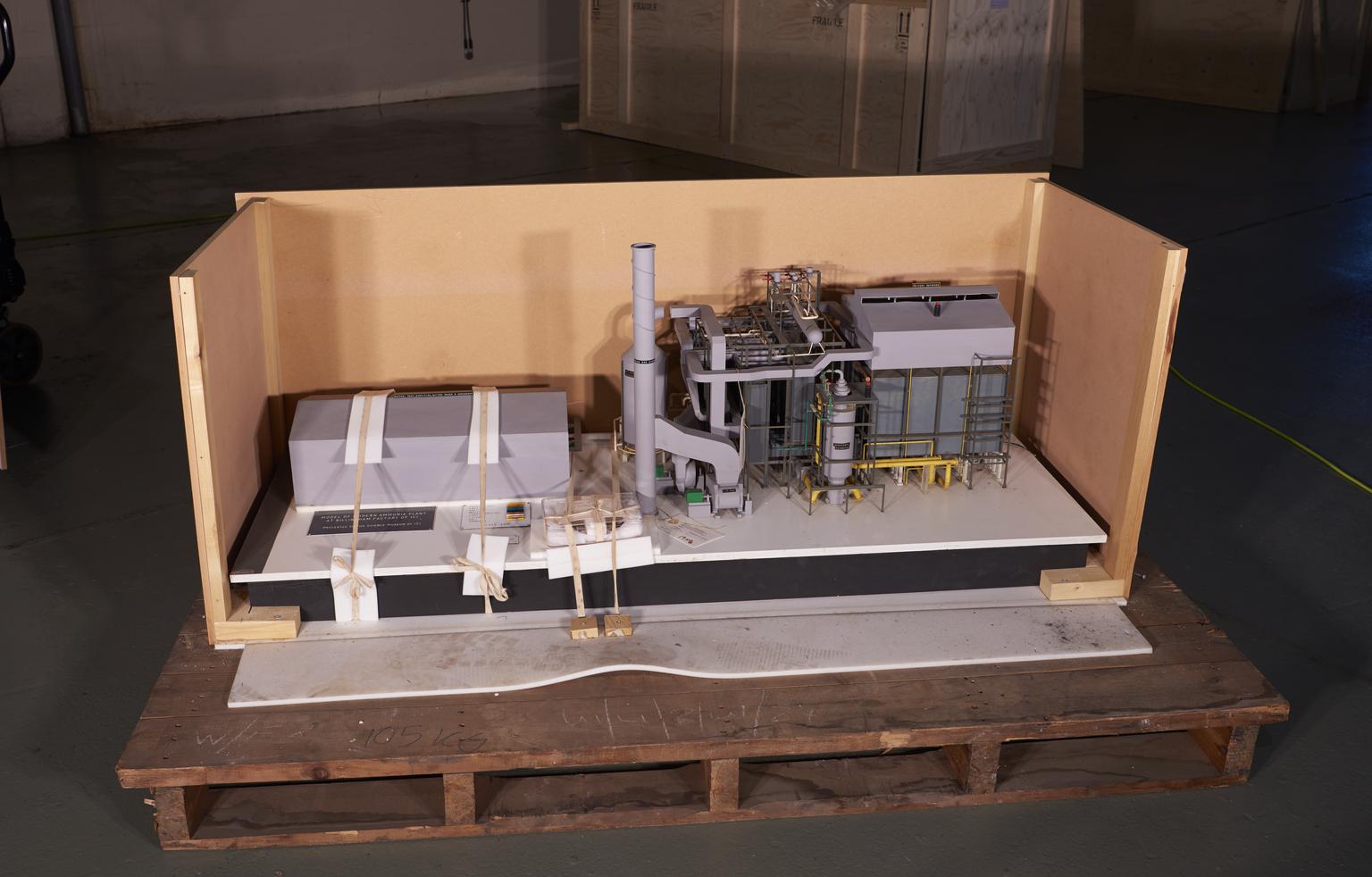
Part of Model of ICI Billingham 'Ammonia Four' Ammonia Synthesis Plant
Part of Model, scale 1:80, of 'Ammonia Four', an ammonia synthesis plant at ICI Agricultural Division, Billingham works, by Cleveland Process Designs and ICI Ltd, Stockton on Tees, England, 1976
More
This model was built in 1976 by Cleveland Process Designs, and depicts ‘Ammonia Four’, the largest of four ammonia plants located at the ICI Billingham works in Stockton-on-Tees, Yorkshire. Ammonia is one of the most important stock chemicals in the modern world, and it is critical precursor for the production of plastics, dyestuffs, explosives, and most crucially fertilizer.
All of ICI’s ammonia plants used the 'Haber-Borsch' process of ammonia synthesis which involved combining three parts hydrogen and one part nitrogen at high pressure and temperature in the presence of a blend of reaction gases most notably being air, steam and a form of hydrocarbon liquid or gas such as naphtha (a by-product of petroleum) or natural methane gas. The ammonia produced in the various ICI Billingham plants was often piped directly to an onsite fertilizer plant which would produce Ammonia nitrate, one of the world's most frequently used fertilizers.
ICI Billingham was initially built in 1918 as the Government Nitrogen factory, before being absorbed into the newly formed Imperial Chemical Industries (ICI) conglomerate. ICI expanded the site in 1960 to have three cutting-edge low-pressure ammonia compression plants which used naphtha as a critical reagent for its ammonia synthesis. The low-pressure design of these plants had the benefit of reducing the costs of running them, improving the efficiency of the ammonia synthesis. ‘Ammonia Four’ was a new generation of low-pressure ammonia plant which made use of natural methane gas (which was cheaper and more plentiful by the 1970s) for its synthesis of ammonia which was designed by ICI in 1973 and made operational by 1977 and continues to operate today. One of the key improvements in the design ‘Ammonia Four’ was its ability to recycle heat energy during the synthesis process, greatly improving the energy efficiency and economy of the ammonia compression process.
The continuous expansion of ICI Billingham during the 20th century reflects the boom in international demand for ammonia for agricultural and industrial purposes. When ICI Billingham first came on stream in 1923, it produced approximately 25 tonnes of synthetic ammonia per day, by 1967 the three new ammonia plants could produce a combined 3000 tonnes a day, and in 1977 the addition of ‘Ammonia Four’ led to a total daily production of ammonia exceeding 4000 tonnes. ICI Billingham could produce around one and half million tonnes (or 1,460,000,000 kgs) of ammonia per year, making it one of the world’s largest producers of ammonia during the 20th and 21st centuries.
The seven buttons on this electric model, when pressed, light up where each stage of the ammonia synthesis takes place in the plant and what reagents, or catalysts are used.
- Measurements:
-
overall: 700 mm x 1570 mm x 600 mm, 28 kg
- Materials:
- paint , wood (unidentified) , plastic (unidentified) , glass and acrylic
- Object Number:
- 1977-269 Pt1
- type:
- model - representation
- Image ©
- The Board of Trustees of the Science Museum